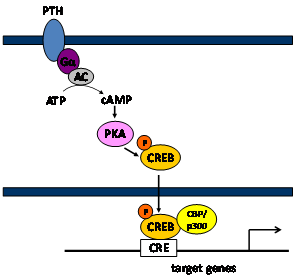
Intermittent application of parathyroid hormone (PTH) has well established anabolic effects on bone mass in rodents and humans. PTH signaling is mediated through 5'-cyclic adenosine monophosphate (cAMP), protein kinase A (PKA), and cAMP response element binding protein (CREB) pathway. PTH binds to a PTH receptor and induces formation of cAMP, leading to activation of PKA, which in turn phosphorylates and activates CREB, a member of a large family of basic leucine zipper (bZIP) domain DNA-binding proteins. Activated CREB, in association with the other co-activators, binds to target genes through a cAMP response element (CRE) and activates their transcription. Therefore, in this pathway, CREB plays a pivotal role by converting the PTH signal to activation of gene expression. However, the transcriptional mechanisms or major downstream targets of CREBtransactivation responsible forthe anabolic effects of PTH on osteoblast differentiation and bone formation need to be elucidated.
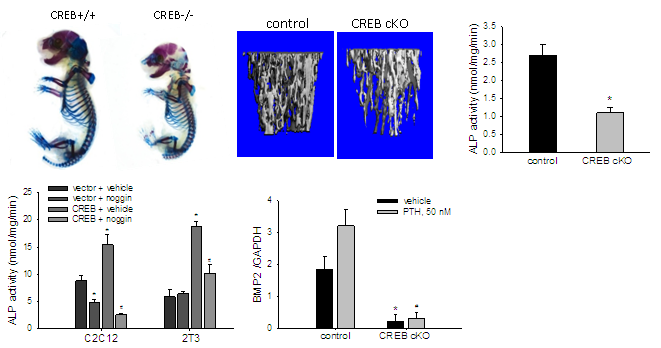
Our in vivo studies on global and osteoblast-specific CREB knockout mice have shown that deficiency of CREB results in significant reduction in bone mass which is caused by inhibition of osteoblast differentiation. In vitro, we found that demonstrated that PTH signaling that activates CREB by phosphorylation promotes osteoblastic differentiation, and that the PTH-CREB signaling pathway functions as an effective activator of BMP2 expression, as pharmacologic and genetic modulation of PTH-CREB activity significantly affects BMP2 transcription through a specific CRE in the BMP2 promoter. These results demonstrate that the anabolic function of PTH signaling in bone is mediated, at least in part, by CREB transactivation of BMP2 expression in osteoblasts. Now, we have an ongoing study to determine the effects of CREB deficiency on the anabolic effects of intermittent administration of PTH on bone mass in the CREB knockout mice.