Hedgehog signaling has been demonstrated important for skeletal development. In vertebrates, the hedgehog signaling is mediated by transcriptional factor Gli proteins, including Gli2 and Gli3, whose activities are regulated by proteasomal processing. In the absence of hedgehog signaling, Gli2 is completely degraded and Gli3 is cleaved into a C' terminal truncated form. When the signaling is initiated, this proteolytic processing of Gli2 and Gli3 is blocked. In vivo and in vitro studies have shown that full-length Gli2 and truncated Gli3 are the predominant functional forms of the Gli family that mediate Hh signaling to target genes.
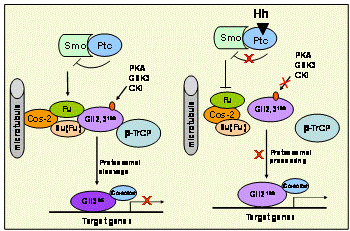
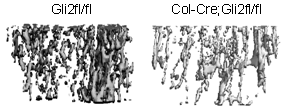
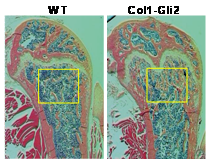
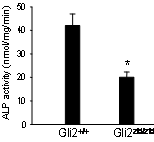
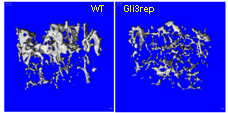
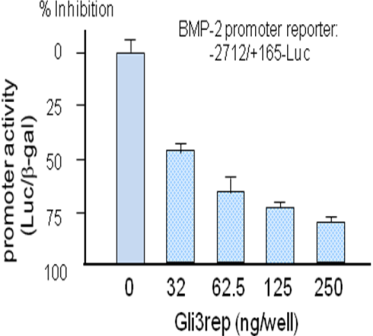
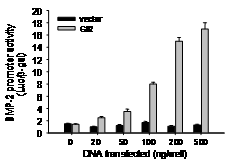
We have demonstrated in vitro that Gli2 and Gli3 are potent regulators of osteoblast differentiation and bone formation. While full-length Gli2 stimulates osteoblast differentiation, truncated Gli3 inhibits osteoblast differentiation and bone formation. Our findings also suggest that the effects of Gli2 and Gli3 on osteoblasts are mediated, at least in part, through BMP2. Full-length Gli2 is a powerful activator of BMP2 transcription, whereas truncated Gli3 acts as a strong BMP2 repressor in osteoblasts. We hence have named the full-length activator Gli2 as Gli2act and the truncated repressor Gli3 as Gli3rep. We hypothesized that Gli2act and Gli3rep play important roles in postnatal bone formation by regulating BMP2 production in osteoblasts, particularly during skeletal aging. Conventional global Gli2 and Gli3 knockout mice do not survive after birth, so we have created osteoblast-specific knockout Gli2act and transgenic Gli3rep mouse models and now we are conducting experiments to test our hypothesis.
 |
 |
 |
 |
 |
 |
