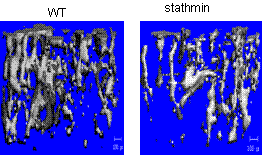
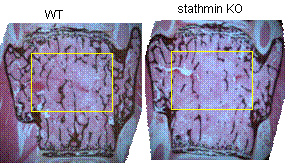
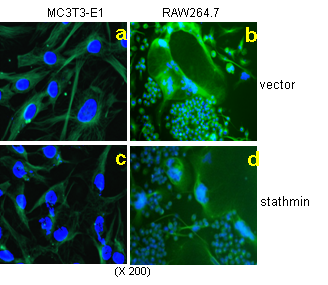
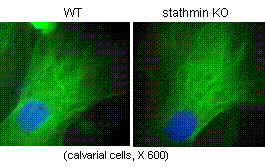
Cytoskeleton microtubules, composed of tubulins, undergo constant assembly and disassembly, modulation of microtubule dynamics affects their biological function. Previously, we have reported that inhibition of microtubule assembly by microtubule-targeting drugs induces BMP2 expression in osteoblasts and consequent bone formation. Stathmin (also referred as OP18), a small cytosolic phosphoprotein, has been identified as a natural endogenous microtubule regulating protein. Stathmin binds directly to microtubules and disrupts their intrinsic dynamic stability by inhibiting assembly and promoting disassembly. We found that stathmin knockout mice develop an osteopenic phenotype low bone mineral density (BMD) and trabecular bone volume. Further characterization showed that the low bone mass is caused by inhibition of osteoblast differentiation and activation of osteoclast formation in bone. In osteoblasts, we found that stathmin KO inhibited Gli2-induced BMP2 transcription. Furthermore, immunofluorescent studies have shown that stathmin functions in both osteoblasts and osteoclasts by affecting microtubule network structure in these cells. These results suggest that microtubule dynamics is potential target for development of novel dual-function drugs that inhibit osteoclastic bone resorption and stimulate osteoblastic bone formation. Currently, we are fully characterizing bone phenotype of stathmin KO mice.
 |
 |
 |
 |
